Facts About Iodine Live Science
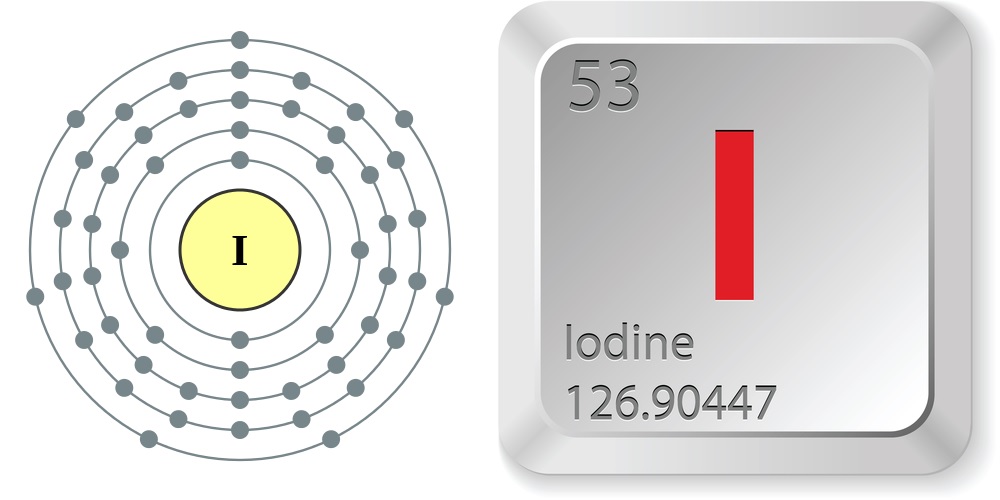
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at 114 °C (237 °F), and boils to a violet gas at 184 °C (363 °F).
Iodine Protons Neutrons Electrons Electron Configuration
0:00 / 2:26 I - Electron Configuration (Iodide Ion) Wayne Breslyn 728K subscribers Subscribe Subscribed 36K views 3 years ago In this video we will write the electron configuration for I- the.

Iodine, atomic structure Stock Image C045/6396 Science Photo Library
In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic table gives the following electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3.
Iodine Photograph by Science Photo Library Fine Art America
Iodine Electron Configuration: Iodine is a chemical element that has a symbol I. The atomic number of Iodine is 53. It is the heaviest of the stable halogens. It exists as a purple-black, lustrous, non-metallic solid at favorable conditions which readily sublimes to form a violet gas. Technetium Valence Electrons.
Iodine atom hires stock photography and images Alamy
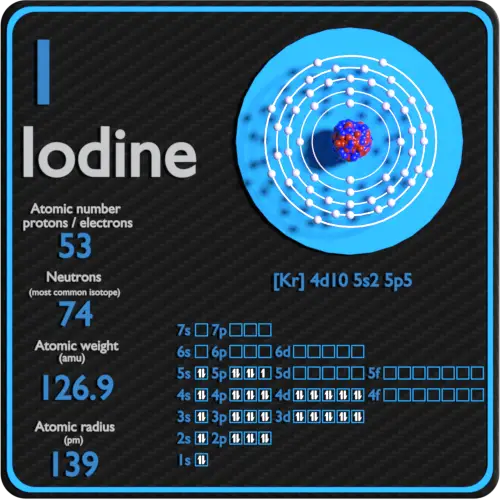
The arrangement of electrons in iodine in specific rules in different orbits and orbitals is called the electron configuration of iodine. The electron configuration of iodine is [ Kr] 4d 10 5s 2 5p 5 , if the electron arrangement is through orbitals. Electron configuration can be done in two ways.
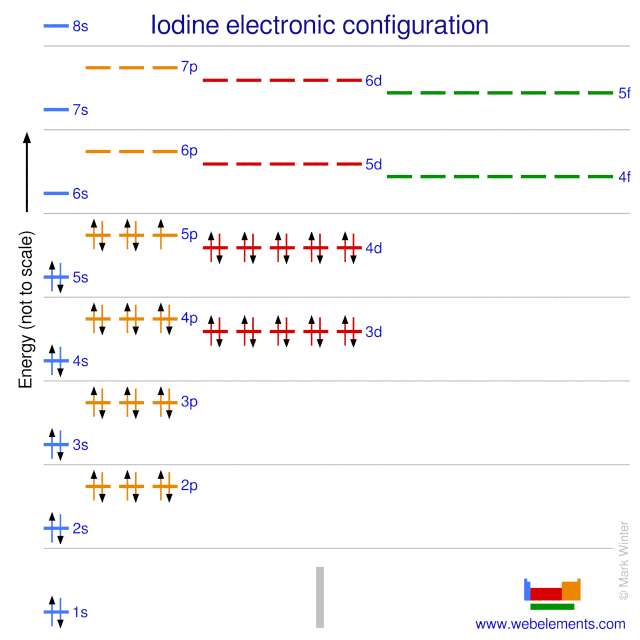
WebElements Periodic Table » Iodine » properties of free atoms
Electron configuration of Iodine is [Kr] 4d10 5s2 5p5. Possible oxidation states are +1,5,7/-1. Electron Configuration The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers, electron configurations, and chemical properties.
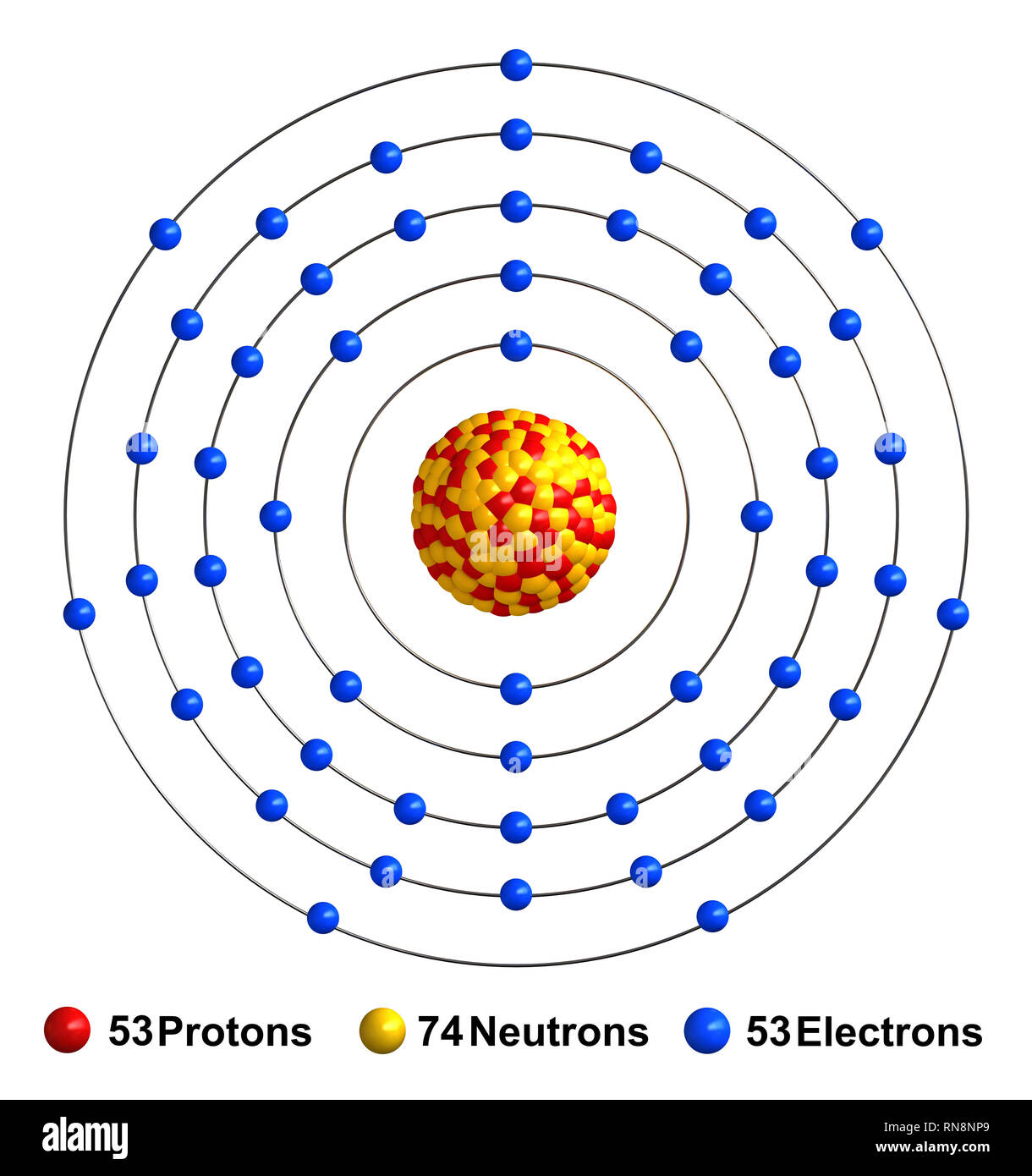
Iodine atom Cut Out Stock Images & Pictures Alamy
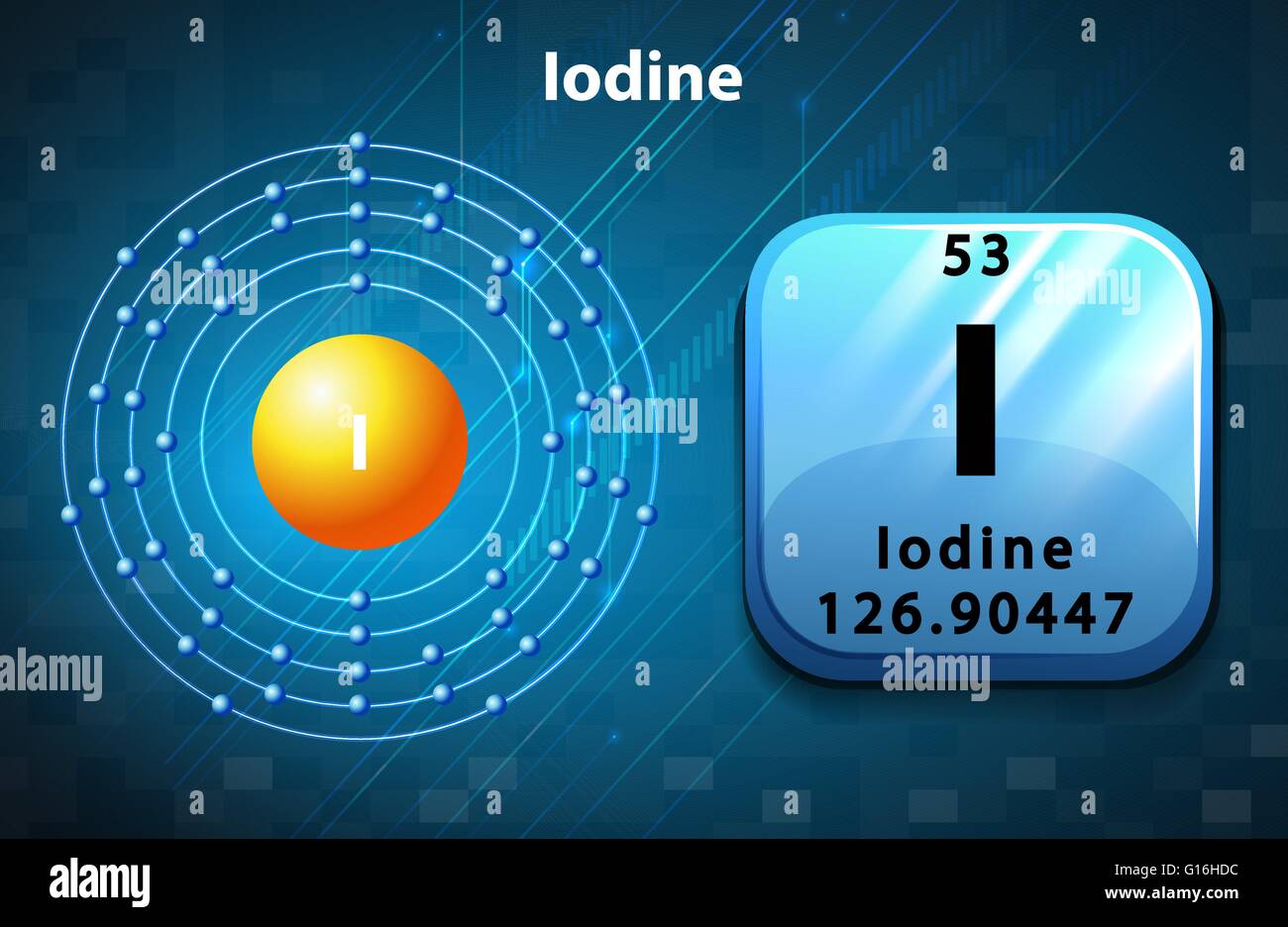
Atomic properties Electron configuration for iodine The history of Iodine Periodic table history Identifiers List of unique identifiers for Iodine in various chemical registry databases Iodine is a chemical element of the periodic table with chemical symbol I and atomic number 53 with an atomic weight of 126.904 u and is classed as a nonmetal.
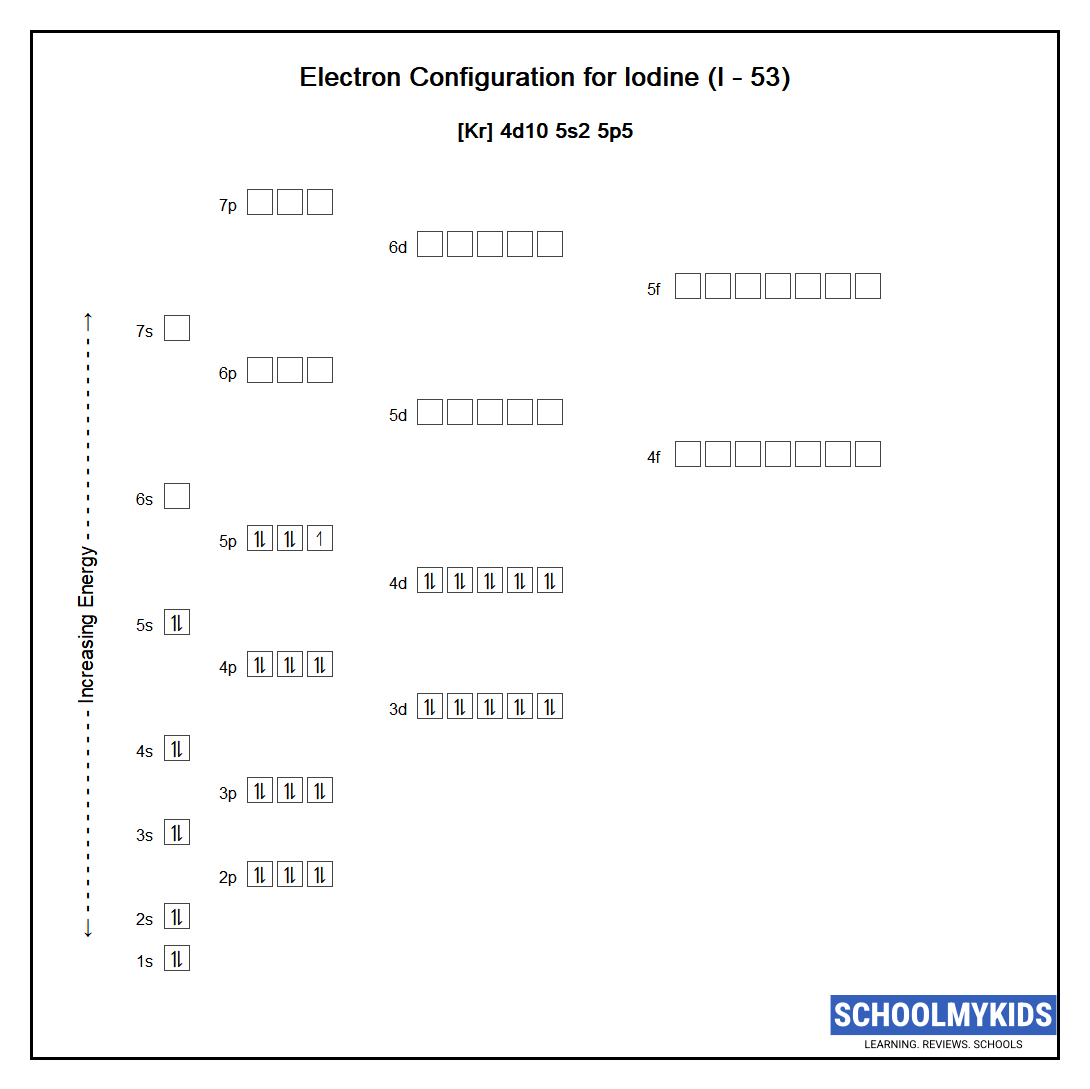
iodine orbital diagram CieranLanna
Configuration. σ g 2 π u 3 π g 3 σ u 2. 7. 4000 torr), are excited by a pulsed high current electron beam Hays, Hoffman, et al. Schwarz, W.H.E., Inner electron excitation of iodine in the gaseous and solid phase, J. Chem. Phys., 1973, 58, 2230. Venkateswarlu, 1970 Venkateswarlu, P., Vacuum ultraviolet spectrum of the iodine molecule.

Lewis Dot Diagram Iodine
Iodine electron configuration. ← Electronic configurations of elements . I (Iodine) is an element with position number 53 in the periodic table. Located in the V period. Melting point: 113.5 ℃. Density: 4.94 g/cm 3. Electronic configuration of the Iodine atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6.

Free Vector Iodine electron configuration atom
Full electron configuration of iodine: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5 tellurium ← iodine → xenon Iodine, complete electron configuration.
How to Find the Valence Electrons for Iodine (I)?
The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction.. (Kr) is the previous noble gas listed before iodine. The noble gas configuration encompases the energy states lower.
Iodine Electron Configuration (I) with Orbital Diagram
Electron configuration: [Kr]4d105s25p5 Oxidation state: ±1,5,7 Crystal structure: orthorhombic . Iodine is a purple-black solid that sublimes at standard temperatures into purple-pink gas with an irritating odor. This element is essential in daily human consumption and is necessary in the production of the thyroid hormone.

Iodine (I) Properties & Uses StudiousGuy
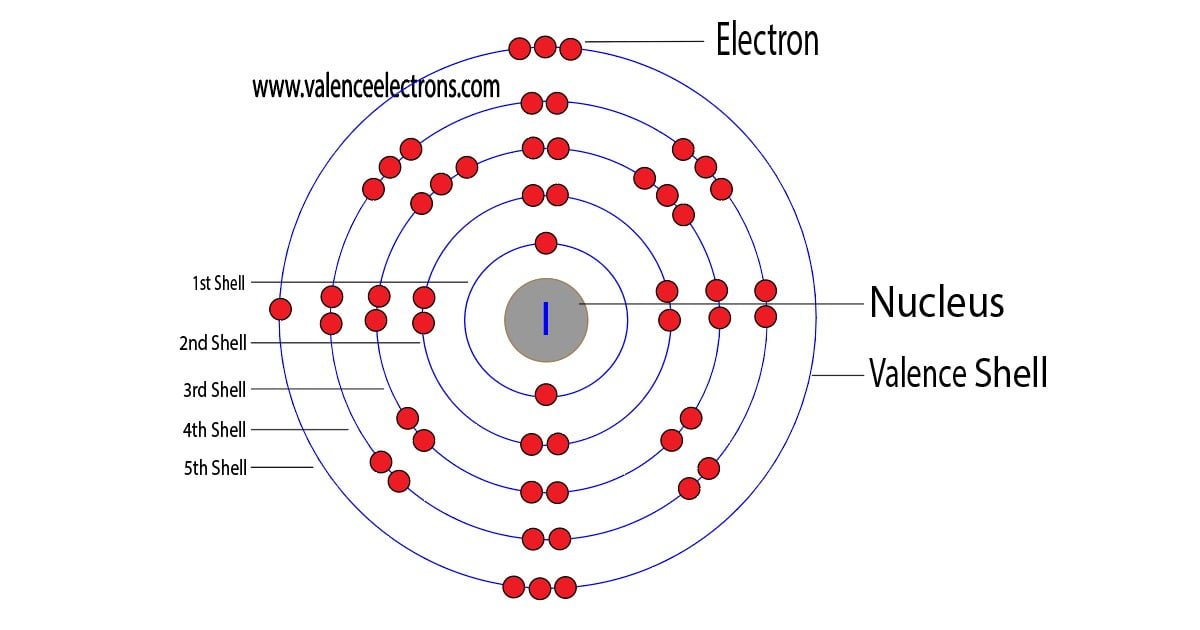
. Electrons in shells Different shells can hold different maximum numbers of electrons. Electrons occupy shells starting with the innermost one. They begin to occupy the next shell when a shell.

Iodine, atomic structure Stock Image C018/3734 Science Photo Library
Electrons revolve around the nucleus in a specific orbit. How to easily find the number of electrons, protons and neutrons in an iodine atom? Scientist Henry Gwynn Jefferies Mosle examined the X-ray spectrum of various elements from 1913 to 1914.

Symbol and electron diagram for iodine Royalty Free Vector
A step-by-step description of how to write the electron configuration for Iodine (I). In order to write the I electron configuration we first need to know t.

PPT Draw Iodine PowerPoint Presentation, free download ID2812883
1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2). You will get the detailed information about the periodic table which will convert a newbie into pro. 3). You will also get the HD images of the Periodic table (for FREE).